http://en.wikipedia.org/wiki/Battery_(electricity)
Battery
A battery is a device that converts chemical energy directly to electrical energy.[22] It consists of a number of voltaic cells; each voltaic cell consists of two half-cells connected in series by a conductive electrolyte containing anions and cations. One half-cell includes electrolyte and the electrode to which anions (negatively charged ions) migrate, i.e., theanode or negative electrode; the other half-cell includes electrolyte and the electrode to which cations (positively charged ions) migrate, i.e., thecathode or positive electrode. In the redox reaction that powers the battery, cations are reduced (electrons are added) at the cathode, while anions are oxidized (electrons are removed) at the anode.[23] The electrodes do not touch each other but are electrically connected by theelectrolyte. Some cells use two half-cells with different electrolytes. A separator between half-cells allows ions to flow, but prevents mixing of the electrolytes.
Each half-cell has an electromotive force (or emf), determined by its ability to drive electric current from the interior to the exterior of the cell. The net emf of the cell is the difference between the emfs of its half-cells, as first recognized by Volta.[12] Therefore, if the electrodes have emfs  and
and  , then the net emf is
, then the net emf is 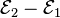 ; in other words, the net emf is the difference between the reduction potentials of the half-reactions.[24]
; in other words, the net emf is the difference between the reduction potentials of the half-reactions.[24]
 and
and  , then the net emf is
, then the net emf is 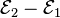 ; in other words, the net emf is the difference between the reduction potentials of the half-reactions.[24]
; in other words, the net emf is the difference between the reduction potentials of the half-reactions.[24]
The electrical driving force or  across the terminals of a cell is known as the terminal voltage (difference) and is measured in volts.[25] The terminal voltage of a cell that is neither charging nor discharging is called the open-circuit voltage and equals the emf of the cell. Because of internal resistance,[26] the terminal voltage of a cell that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a cell that is charging exceeds the open-circuit voltage.[27] An ideal cell has negligible internal resistance, so it would maintain a constant terminal voltage of
across the terminals of a cell is known as the terminal voltage (difference) and is measured in volts.[25] The terminal voltage of a cell that is neither charging nor discharging is called the open-circuit voltage and equals the emf of the cell. Because of internal resistance,[26] the terminal voltage of a cell that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a cell that is charging exceeds the open-circuit voltage.[27] An ideal cell has negligible internal resistance, so it would maintain a constant terminal voltage of 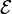 until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one coulomb then on complete discharge it would perform 1.5 joule of work.[25] In actual cells, the internal resistance increases under discharge,[26] and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a curve; the shape of the curve varies according to the chemistry and internal arrangement employed.[28]
until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one coulomb then on complete discharge it would perform 1.5 joule of work.[25] In actual cells, the internal resistance increases under discharge,[26] and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a curve; the shape of the curve varies according to the chemistry and internal arrangement employed.[28]
 across the terminals of a cell is known as the terminal voltage (difference) and is measured in volts.[25] The terminal voltage of a cell that is neither charging nor discharging is called the open-circuit voltage and equals the emf of the cell. Because of internal resistance,[26] the terminal voltage of a cell that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a cell that is charging exceeds the open-circuit voltage.[27] An ideal cell has negligible internal resistance, so it would maintain a constant terminal voltage of
across the terminals of a cell is known as the terminal voltage (difference) and is measured in volts.[25] The terminal voltage of a cell that is neither charging nor discharging is called the open-circuit voltage and equals the emf of the cell. Because of internal resistance,[26] the terminal voltage of a cell that is discharging is smaller in magnitude than the open-circuit voltage and the terminal voltage of a cell that is charging exceeds the open-circuit voltage.[27] An ideal cell has negligible internal resistance, so it would maintain a constant terminal voltage of 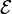 until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one coulomb then on complete discharge it would perform 1.5 joule of work.[25] In actual cells, the internal resistance increases under discharge,[26] and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a curve; the shape of the curve varies according to the chemistry and internal arrangement employed.[28]
until exhausted, then dropping to zero. If such a cell maintained 1.5 volts and stored a charge of one coulomb then on complete discharge it would perform 1.5 joule of work.[25] In actual cells, the internal resistance increases under discharge,[26] and the open circuit voltage also decreases under discharge. If the voltage and resistance are plotted against time, the resulting graphs typically are a curve; the shape of the curve varies according to the chemistry and internal arrangement employed.[28]
As stated above, the voltage developed across a cell's terminals depends on the energy release of the chemical reactions of its electrodes and electrolyte. Alkaline and zinc–carbon cells have different chemistries but approximately the same emf of 1.5 volts; likewise NiCd and NiMH cells have different chemistries, but approximately the same emf of 1.2 volts.[29] On the other hand the high electrochemical potential changes in the reactions of lithium compounds give lithium cells emfs of 3 volts or more.[30]
Categories and types of batteries
Main article: List of battery types
Batteries are classified into two broad categories, each type with advantages and disadvantages.[31]
- Primary batteries irreversibly (within limits of practicality) transform chemical energy to electrical energy. When the initial supply of reactants is exhausted, energy cannot be readily restored to the battery by electrical means.[32]
- Secondary batteries can be recharged; that is, they can have their chemical reactions reversed by supplying electrical energy to the cell, restoring their original composition.[33]
Some types of primary batteries used, for example, for telegraph circuits, were restored to operation by replacing the components of the battery consumed by the chemical reaction.[34] Secondary batteries are not indefinitely rechargeable due to dissipation of the active materials, loss of electrolyte and internal corrosion.
Primary batteries
Main article: Primary cell
Primary batteries can produce current immediately on assembly. Disposable batteries are intended to be used once and discarded. These are most commonly used in portable devices that have low current drain, are used only intermittently, or are used well away from an alternative power source, such as in alarm and communication circuits where other electric power is only intermittently available. Disposable primary cells cannot be reliably recharged, since the chemical reactions are not easily reversible and active materials may not return to their original forms. Battery manufacturers recommend against attempting to recharge primary cells.[35]
Common types of disposable batteries include zinc–carbon batteries and alkaline batteries. In general, these have higher energy densities than rechargeable batteries,[36] but disposable batteries do not fare well under high-drain applications with loads under 75 ohms (75 Ω).[31]
Secondary batteries
Main article: Rechargeable battery
Secondary batteries must be charged before use; they are usually assembled with active materials in the discharged state. Rechargeable batteries orsecondary cells can be recharged by applying electric current, which reverses thechemical reactions that occur during its use. Devices to supply the appropriate current are called chargers or rechargers.
The oldest form of rechargeable battery is the lead–acid battery.[37] This battery is notable in that it contains a liquid in an unsealed container, requiring that the battery be kept upright and the area be well ventilated to ensure safe dispersal of the hydrogen gas produced by these batteries during overcharging. The lead–acid battery is also very heavy for the amount of electrical energy it can supply. Despite this, its low manufacturing cost and its high surge current levels make its use common where a large capacity (over approximately 10 Ah) is required or where the weight and ease of handling are not concerns.
A common form of the lead–acid battery is the modern car battery, which can, in general, deliver a peak current of 450 amperes.[38] An improved type of liquid electrolyte battery is the sealed valve regulated lead–acid battery (VRLA battery), popular in the automotive industry as a replacement for the lead–acid wet cell. The VRLA battery uses an immobilized sulfuric acid electrolyte, reducing the chance of leakage and extending shelf life.[39] VRLA batteries have the electrolyte immobilized, usually by one of two means:
- Gel batteries (or "gel cell") contain a semi-solid electrolyte to prevent spillage.
- Absorbed Glass Mat (AGM) batteries absorb the electrolyte in a special fiberglass matting.
Other portable rechargeable batteries include several "dry cell" types, which are sealed units and are, therefore, useful in appliances such as mobile phones and laptop computers. Cells of this type (in order of increasing power density and cost) include nickel–cadmium (NiCd), nickel–zinc (NiZn), nickel metal hydride (NiMH), and lithium-ion(Li-ion) cells.[40] By far, Li-ion has the highest share of the dry cell rechargeable market.[3] Meanwhile, NiMH has replaced NiCd in most applications due to its higher capacity, but NiCd remains in use in power tools, two-way radios, and medical equipment.[3] NiZn is a new technology that is not yet well established commercially.
Recent developments include batteries with embedded electronics such as USBCELL, which allows charging an AA cell through a USB connector,[41] and smart battery packs with state-of-charge monitors and battery protection circuits to prevent damage on over-discharge. low self-discharge (LSD) allows secondary cells to be precharged prior to shipping.
Battery cell types
There are many general types of electrochemical cells, according to chemical processes applied and design chosen. The variation includes galvanic cells, electrolytic cells, fuel cells, flow cells and voltaic piles.[42]
Wet cell
A wet cell battery has a liquid electrolyte. Other names are flooded cell, since the liquid covers all internal parts, orvented cell, since gases produced during operation can escape to the air. Wet cells were a precursor to dry cells and are commonly used as a learning tool for electrochemistry. It is often built with common laboratory supplies, such as beakers, for demonstrations of how electrochemical cells work. A particular type of wet cell known as aconcentration cell is important in understanding corrosion. Wet cells may be primary cells (non-rechargeable) orsecondary cells (rechargeable). Originally, all practical primary batteries such as the Daniell cell were built as open-topped glass jar wet cells. Other primary wet cells are the Leclanche cell, Grove cell, Bunsen cell, Chromic acid cell, Clark cell, and Weston cell. The Leclanche cell chemistry was adapted to the first dry cells. Wet cells are still used in automobile batteries and in industry for standby power for switchgear, telecommunication or largeuninterruptible power supplies, but in many places batteries with gel cells have been used instead. These applications commonly use lead–acid or nickel–cadmium cells.
Dry cell
"Dry cell" redirects here. For the heavy metal band, see Dry Cell (band).
A dry cell has the electrolyte immobilized as a paste, with only enough moisture in it to allow current to flow. Unlike a wet cell, a dry cell can operate in any orientation without spilling as it contains no free liquid, making it suitable for portable equipment. By comparison, the first wet cells were typically fragile glass containers with lead rods hanging from the open top, and needed careful handling to avoid spillage. Lead–acid batteries did not achieve the safety and portability of the dry cell until the development of the gel battery.
A common dry cell battery is the zinc–carbon battery, using a cell sometimes called the dry Leclanché cell, with a nominal voltage of 1.5 volts, the same as thealkaline battery (since both use the same zinc–manganese dioxide combination).
A standard dry cell comprises a zinc anode (negative pole), usually in the form of a cylindrical pot, with a carbon cathode (positive pole) in the form of a central rod. The electrolyte is ammonium chloride in the form of a paste next to the zinc anode. The remaining space between the electrolyte and carbon cathode is taken up by a second paste consisting of ammonium chloride and manganese dioxide, the latter acting as a depolariser. In some more modern types of so-called 'high-power' batteries (with much lower capacity than standard alkaline batteries), the ammonium chloride is replaced by zinc chloride.
Molten salt
Molten salt batteries are primary or secondary batteries that use a molten salt as electrolyte. Their energy densityand power density give them potential for use in electric vehicles, but they operate at high temperatures and must be well insulated to retain heat.
Reserve
A reserve battery is stored in unassembled form and is activated, ready-charged, when its internal parts are assembled, e.g. by adding electrolyte; it can be stored unactivated for a long period of time. For example, a battery for an electronic fuze might be activated by the impact of firing a gun, breaking a capsule of electrolyte to activate the battery and power the fuze's circuits. Reserve batteries are usually designed for a short service life (seconds or minutes) after long storage (years). A water-activated battery for oceanographic instruments or military applications becomes activated on immersion in water.
Battery cell performance
A battery's characteristics may vary over load cycle, over charge cycle, and over lifetime due to many factors including internal chemistry, current drain, and temperature.


I would be very keen if you could do a follow up article about ups power suppliers batteries differ. Thank you for the article none the less.
ReplyDeleteHi Jay Shenton. Glad to hear from you. Sure will post an article about the mentioned. All post will be happily responded within 24 hours time. Kindly recommend the article to your friends. Thank you
Delete